Abstract
Introduction: DLBCL is an aggressive form of non-Hodgkin lymphoma (NHL) occurring more frequently in older patients (pts). Frontline treatment usually consists of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), which is curative in roughly 60% of pts. However, around 30% of pts relapse, and ~ 10% are refractory to treatment. Pts with R/R DLBCL have a poor prognosis with a median overall survival (OS) of ~ 10 months (mo). Second-line therapy involves high-dose chemotherapy and stem cell transplantation (SCT); however, this is not suitable for all pts, in particular those who are elderly or have comorbidities. While the SCHOLAR-1 study recently highlighted the need for effective therapies in pts with refractory DLBCL, little is known about the unmet needs in pts with relapsed DLBCL. These 3 SLRs were carried out to determine the clinical, economic, and disease burden among pts with relapsed and refractory DLBCL.
Methods: Clinical and economic SLRs were conducted in accordance with European Union Health Technology Assessment (EU HTA) requirements (2 independent reviewers and quality assessment). The clinical SLR identified randomized and non-randomized/observational studies from EMBASE, MEDLINE, The Cochrane Library, and clinical conferences (ASCO, ESMO, EHA, ASH, ICML, AACR, and EORTC) from Jan 2000 to Oct 2017. The economic SLR identified economic evidence from EMBASE, MEDLINE, PsycINFO, The Cochrane Library, ISPOR, clinical conferences, and HTA websites from Jan 2000 to Apr 2017. To evaluate the burden of disease in R/R DLBCL, a SLR (1 reviewer) was carried out to identify publications from EMBASE, MEDLINE, and PsycINFO from Jan 2007 to Oct 2017. All 3 SLRs were performed according to the Cochrane Handbook for Systematic Reviews of Interventions and PRISMA guidelines.
Results: The 3 SLRs identified 60, 25, and 39 studies for the clinical, economic, and disease burden reviews, respectively. While DLBCL is a highly heterogeneous diagnostic category, most publications reported on R/R DLBCL pts in general and did not specify prognosis or disease burden for individual pt subsets. Few studies provided separate analyses for primary relapse and subsequent relapse. R/R DLBCL was common with 25% of pts progressing ~ 1 year after 1st-line R-CHOP and 44% progressing ~ 1 year after SCT. Older pts and those with non-germinal center B-cell DLBCL were more likely to relapse.
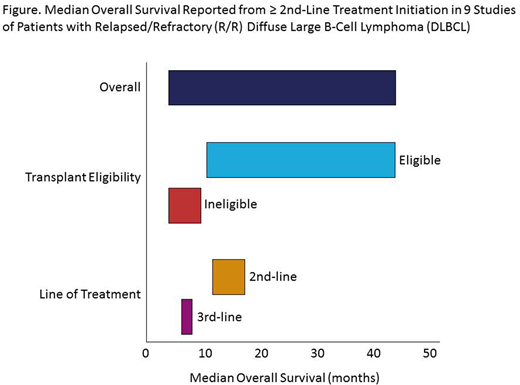
The clinical SLR found a large unmet need in R/R DLBCL due to limited availability of effective treatments. Wide variations in OS outcomes for R/R DLBCL pts were observed, dependent on pt characteristics and line of treatment (Figure). In studies including pts eligible for transplantation, median OS was 9.9-44.0 mo, whereas observed OS was shorter in pts ineligible for transplantation (3.4-9.0 mo). For pts receiving 3rd-line salvage therapy, OS was also shorter compared with 2nd-line salvage therapy, ranging from 5.9 to 8.0 mo vs 11 to 17.2 mo.
The economic SLR identified limited information (4 studies) on the economic burden of several ≥ 2nd-line R/R DLBCL treatments including SCT. The reported incremental cost-effectiveness ratios (ICERs) ranged between GBP 11,018 and GBP 45,282 per QALY gained for plerixafor, mini-BEAM (carmustine, etoposide, cytarabine, melphalan), and pixantrone, though there was considerable heterogeneity of studies in treatment or perspective. Direct costs for 2nd-line treatment were primarily driven by autologous SCT and hospitalization costs, followed by drug costs.
The disease burden SLR found the most common symptoms reported were fever, night sweats, and weight loss. Health-related quality of life (HRQoL) in R/R DLBCL was lowest after the 1st treatment cycle and was impacted by treatment-related adverse events. After SCT, it was shown that few pts (35%) returned to work, 14% retired, and 23% died. Currently, there are no HRQoL instruments specific for DLBCL.
Conclusions: This SLR of studies in pts with relapsed and refractory DLBCL performed prior to CAR T cell therapy introduction suggests that the largest unmet need in R/R DLBCL remains effective treatment options, particularly in pts ineligible for SCT. The short- and long-term economic burden of R/R DLBCL is substantial, largely due to the cost of autologous SCT, hospitalizations, and drugs. HRQoL tools specific for DLBCL would facilitate the accurate determination of the disease burden.
Lenz:Celgene Corp.: Consultancy, Honoraria, Other: Travel, Accomodations, Expenses, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria; Novartis: Research Funding; Bayer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Other: Travel, Accomodations, Expenses, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accomodations, Expenses, Research Funding, Speakers Bureau. Davies:GSK: Research Funding; Pfizer: Research Funding; Gilead: Honoraria, Research Funding; Karyopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; Kite: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; F. Hoffman-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Zinzani:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bayer: Membership on an entity's Board of Directors or advisory committees; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Speakers Bureau; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau. Salles:Epizyme: Honoraria; Celgene: Honoraria, Other: Advisory Board, Research Funding; Novartis: Consultancy, Honoraria; Acerta: Honoraria; Servier: Honoraria, Other: Advisory Board; Merck: Honoraria; Takeda: Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Gilead: Honoraria, Other: Advisory Board; Morphosys: Honoraria; BMS: Honoraria, Other: Advisory Board; Janssen: Honoraria, Other: Advisory Board; Servier: Honoraria; Pfizer: Honoraria; Abbvie: Honoraria; Amgen: Honoraria. Hasskarl:Celgene Corp.: Employment, Equity Ownership. Margunato-Debay:Celgene Corp.: Employment, Equity Ownership. Rodrigues:Celgene Corp.: Employment. Nientker:Celgene Corp.: Consultancy. Balradj:Celgene Corp.: Consultancy. Alleman:Celgene Corp.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.